Real-World Evidence as a Trailblazer of Data Medicine
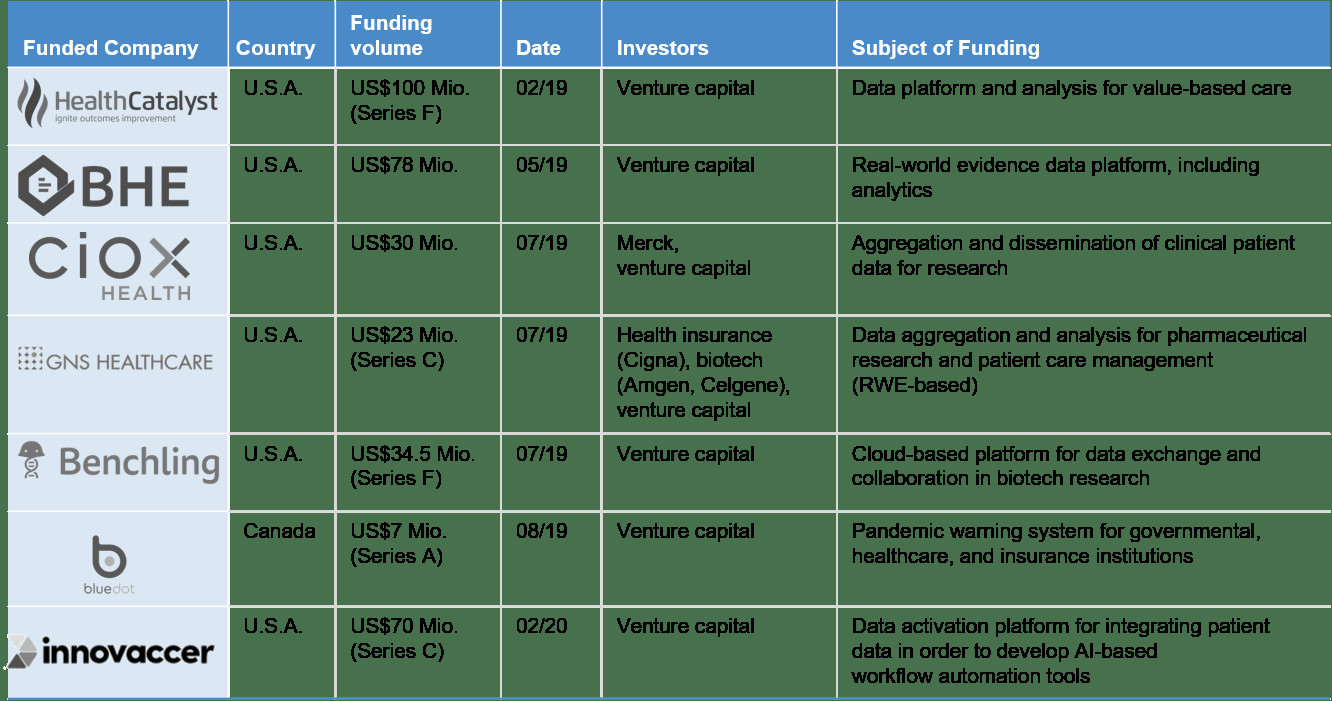
Real-world evidence – in Germany and Europe, this development is still in its beginning. With our clients at Statista, we are working with are working to change that.


An additional example is the oncological real-world evidence platform Flatiron Health, which was acquired by Roche for USD1.9 billion in 2018.
What is the reason for these high valuations? The aforementioned platforms follow different business models—from pure data collection and aggregation, to solutions for data swapping, to software tools for customized patient therapy in cancer centers. Their common ground is the aspiration to reduce costs in developing medications and to increase the accuracy of fit in therapies by using real-world data. In the long term, the use of machine learning and AI will also play an important role. The following sketch illustrates this approach for the pharmaceutical industry:
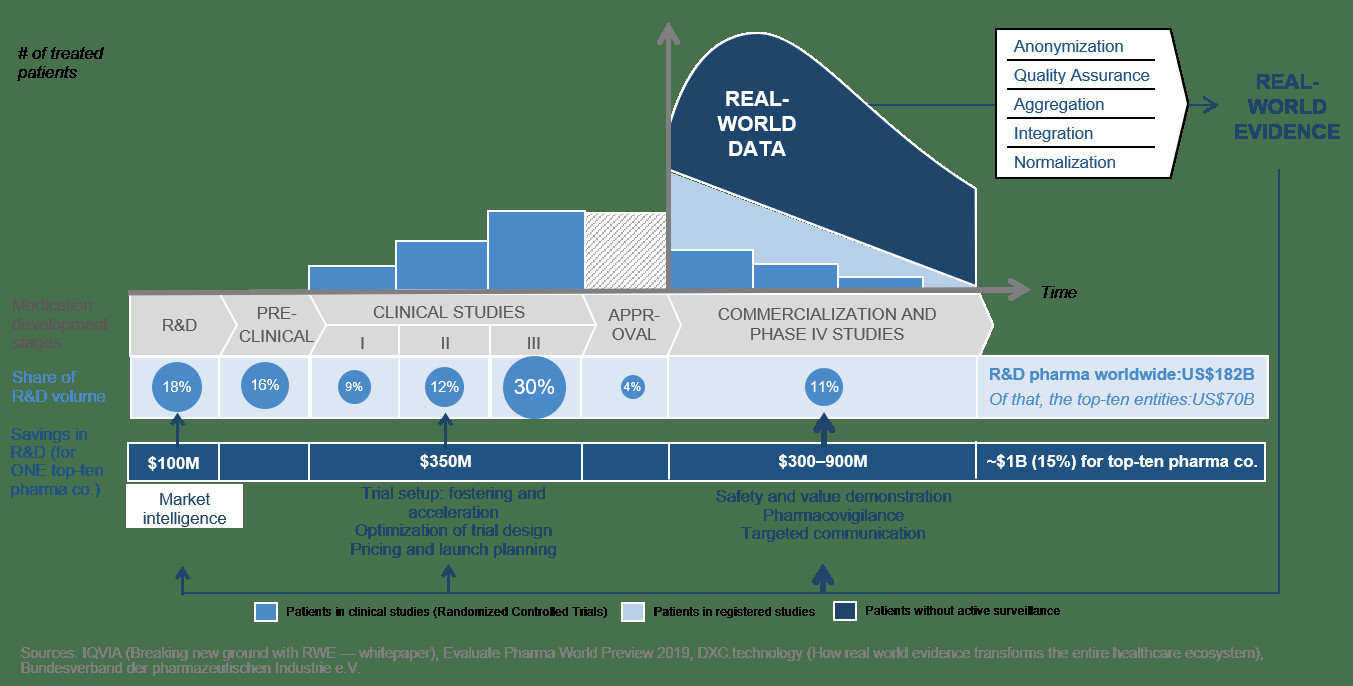
As early as 2016, IQVIA calculated savings of 15% in pharmaceutical research using real-world evidence would add up to a worldwide total of more than US$27 billion.
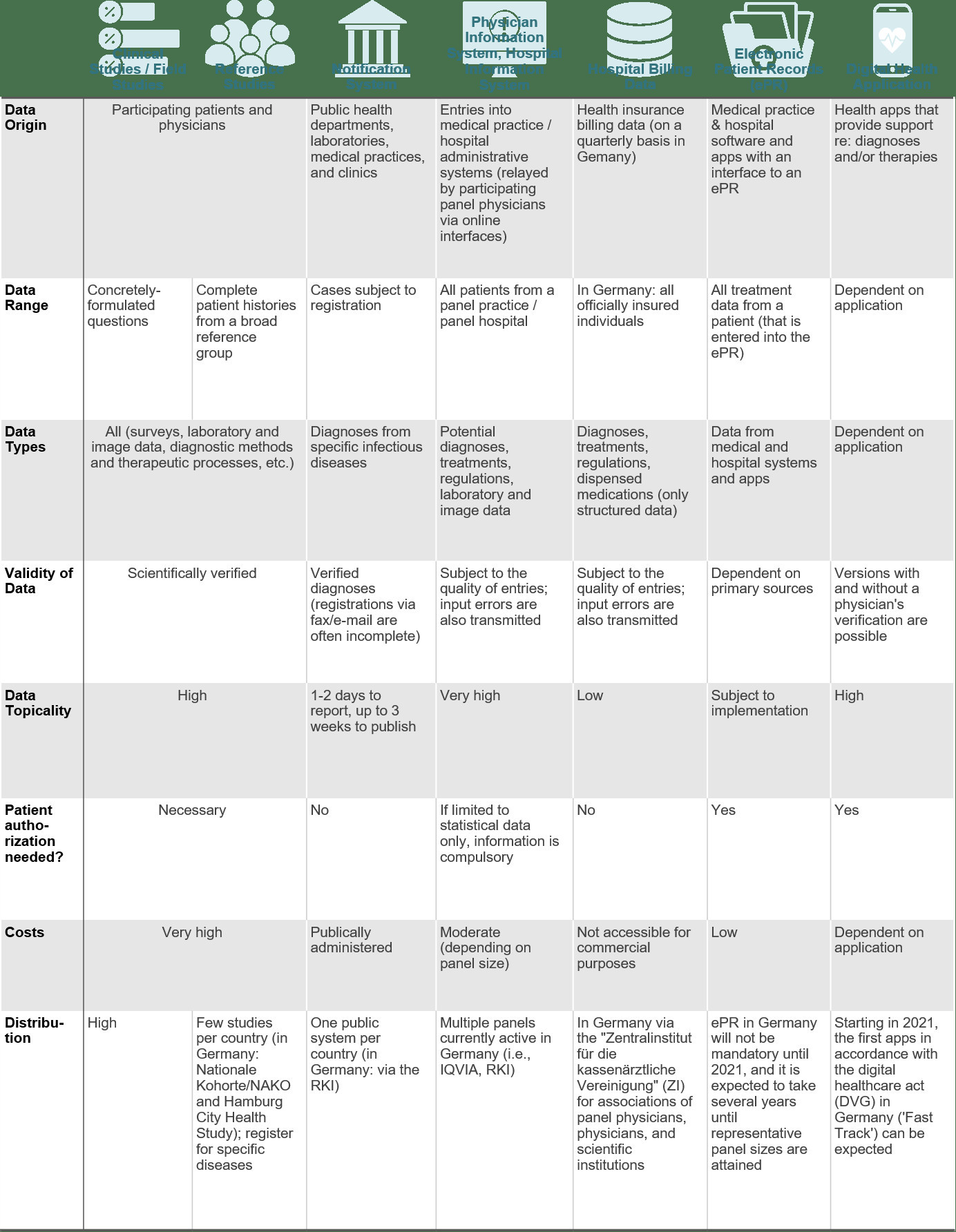
Sources of „Real World Evidence“
In comparison to the U.S.A., the availability of real-world evidence in Germany remains quite restricted. In Germany, for example, the electronic patient file (which has been mandatory in the USA with the HiTECH Act since 2009) will not be introduced until next year. For this reason, the „Zentralinstitut für die Kassenärztlichen Vereinigungen“ (ZI) only provides billing data of public health insurance companies to physicians and scientific research institutions (with a considerable time delay).
With the introduction of electronic patient files and digital health applications as well as the comprehensive rollout of the teleinformatic infrastructure, this situation will change in Germany in the years ahead. It is already possible to construct physician panels by way of plug-ins into medical practice management software and obtain data about specific patient groups using digital health applications. But even here, we have to contend with a time requirement of one to two years until a representative amount of data has been achieved. You can find an overview of the most important potential data sources for real-world evidence here:
Beginnings of Real-World Evidence Platforms in Germany
In Germany, the setup of real-world evidence platforms primarily belongs to the world of university research. In addition to the national longitudinal study (NAKO Gesundheitsstudie), the data integration centers of the Medical Informatics Initiative of the Federal Ministry for Education and Research, or BMBF as well as the „Hamburg City Health Study“ (HCHS) are especially worth mentioning here. Here, the focus resides primarily in the preservation of data in a clinical and/or in-patient context The chief private provider of real-world evidence data is currently the American company IQVIA, which runs a panel with several thousand physicians in clinical as well as in-patient sectors. Yet at the moment, this situation is changing, and since 2019, increasing numbers of initiatives are coming into existence, in Germany as well, to set up real-world evidence platforms. Among them are:
- - Data4Life: A charitable initiaitve of the Hasso Plattner Institute. At the moment, the focus is on the development of a European solution for distributing and collecting health data within the framework of the 'Smart4Life' consortium.
- - Urogista / Uroscience: Initiative of the 'Deutsches Institut für fachärztliche Versorgungsforschung' (DIFA) and the 'Berufsverband der Deutschen Urologen e.V.' (Professional Association of German Urologists) to automate notifications for the cancer registry via 'plug-ins' into the medical practice management system, store them in a central database, and with that said, facilitate the participation of urologists in studies and health care research projects.
- Docmetric: Initiative of CompuGroup Medical AG to use the CGM medical practice management systems to obtain real-time statistical information on diagnoses and treatments.
Contact us if you also wish to tap into the revenue and efficiency potentialities of real-world evidence for your business.
Let’s speak about what we can do for you
How can we help you?



